Preethika Muralidharan
RetinaRegNet: A Versatile Approach for Retinal Image Registration
Apr 24, 2024



Abstract:We introduce the RetinaRegNet model, which can achieve state-of-the-art performance across various retinal image registration tasks. RetinaRegNet does not require training on any retinal images. It begins by establishing point correspondences between two retinal images using image features derived from diffusion models. This process involves the selection of feature points from the moving image using the SIFT algorithm alongside random point sampling. For each selected feature point, a 2D correlation map is computed by assessing the similarity between the feature vector at that point and the feature vectors of all pixels in the fixed image. The pixel with the highest similarity score in the correlation map corresponds to the feature point in the moving image. To remove outliers in the estimated point correspondences, we first applied an inverse consistency constraint, followed by a transformation-based outlier detector. This method proved to outperform the widely used random sample consensus (RANSAC) outlier detector by a significant margin. To handle large deformations, we utilized a two-stage image registration framework. A homography transformation was used in the first stage and a more accurate third-order polynomial transformation was used in the second stage. The model's effectiveness was demonstrated across three retinal image datasets: color fundus images, fluorescein angiography images, and laser speckle flowgraphy images. RetinaRegNet outperformed current state-of-the-art methods in all three datasets. It was especially effective for registering image pairs with large displacement and scaling deformations. This innovation holds promise for various applications in retinal image analysis. Our code is publicly available at https://github.com/mirthAI/RetinaRegNet.
MicroSegNet: A Deep Learning Approach for Prostate Segmentation on Micro-Ultrasound Images
May 31, 2023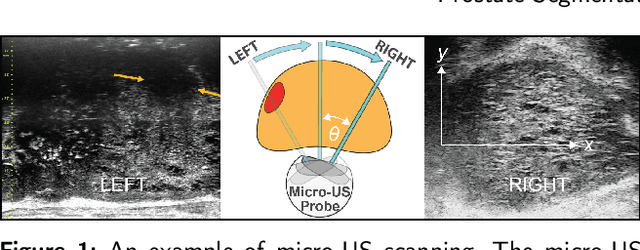
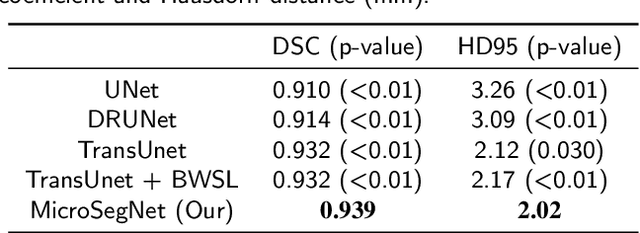
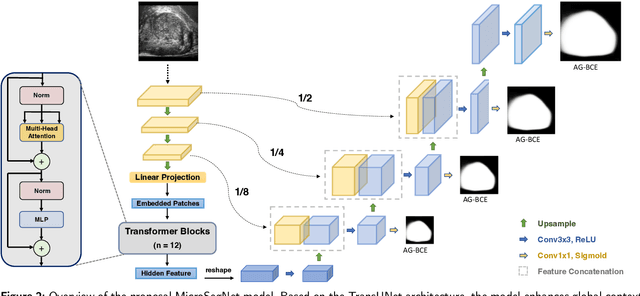
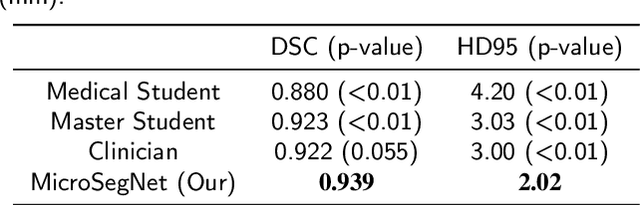
Abstract:Micro-ultrasound (micro-US) is a novel 29-MHz ultrasound technique that provides 3-4 times higher resolution than traditional ultrasound, delivering comparable accuracy for diagnosing prostate cancer to MRI but at a lower cost. Accurate prostate segmentation is crucial for prostate volume measurement, cancer diagnosis, prostate biopsy, and treatment planning. This paper proposes a deep learning approach for automated, fast, and accurate prostate segmentation on micro-US images. Prostate segmentation on micro-US is challenging due to artifacts and indistinct borders between the prostate, bladder, and urethra in the midline. We introduce MicroSegNet, a multi-scale annotation-guided Transformer UNet model to address this challenge. During the training process, MicroSegNet focuses more on regions that are hard to segment (challenging regions), where expert and non-expert annotations show discrepancies. We achieve this by proposing an annotation-guided cross entropy loss that assigns larger weight to pixels in hard regions and lower weight to pixels in easy regions. We trained our model using micro-US images from 55 patients, followed by evaluation on 20 patients. Our MicroSegNet model achieved a Dice coefficient of 0.942 and a Hausdorff distance of 2.11 mm, outperforming several state-of-the-art segmentation methods, as well as three human annotators with different experience levels. We will make our code and dataset publicly available to promote transparency and collaboration in research.
 Add to Chrome
Add to Chrome Add to Firefox
Add to Firefox Add to Edge
Add to Edge